What is PDN?
There are over 34,000,000 people in the United States with diabetes. That’s about 1 every 10 people. For some of those people, painful diabetic neuropathy (PDN) can lead to the development of numbness, burning, tingling and cold in the lower legs and feet. This is sometimes described as a “stocking distribution” of symptoms. Until now, PDN has had limited treatment options.
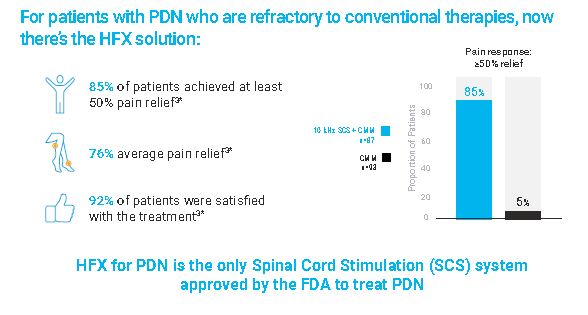
Recently, a randomized, controlled study (RCT) was performed in 216 patients suffering from PDN. The study demonstrated that 85% of patients receiving HFX therapy achieved 50% or greater pain relief compared to only 5% of patient reporting relief with medications alone. The HFX patients also reported improvement in sleep as well as quality of life as a result of HFX therapy.
What is HFX Therapy?
HFX stands for “High Frequency, 10 kHz”. This refers to a high-frequency spinal cord stimulating device that has been used in over 70,000 patients suffering from chronic back and leg pain due to spinal pathology. The high-frequency stimulation allows patients to achieve pain relief without the tingling that is typically felt with traditional spinal cord stimulation.
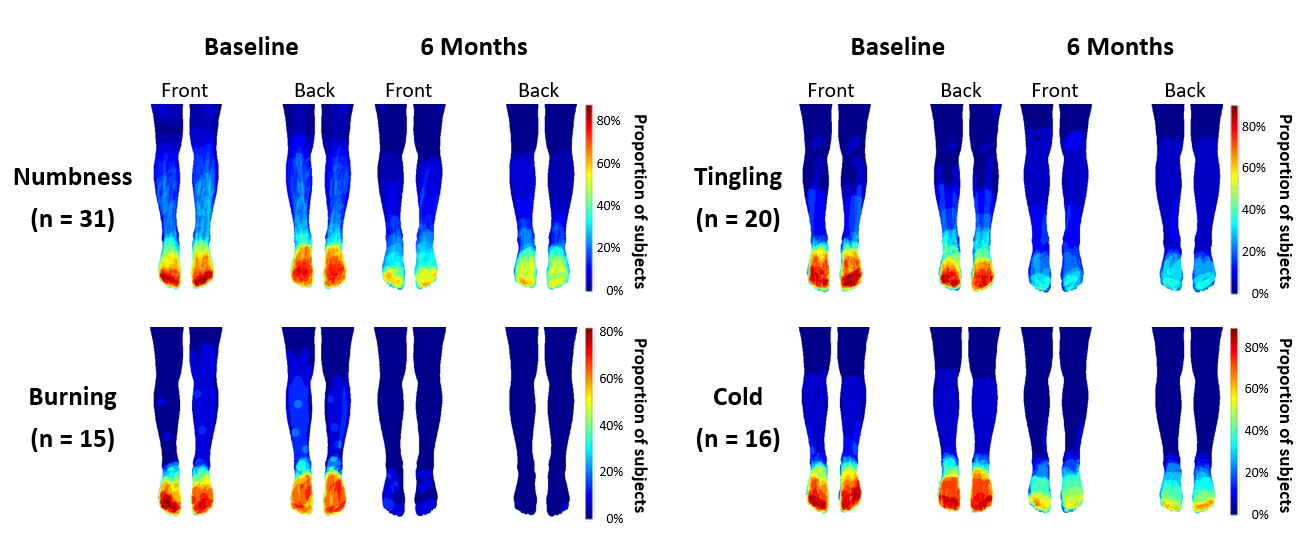
A Picture is Worth a Thousand Words
The graphic below shows a visual representation of improvements in numbness, burning, tingling and cold reported by patients receiving HFX therapy. Lower amounts of red represents a lower degree of symptoms.
Is HFX FDA approved? Is it covered by my Insurance?
HFX is an FDA-approved therapy and is covered by most insurance programs. In order to qualify for HFX therapy, patients must meet the following criteria:
- Be diagnosed with and treated for diabetes. Blood sugar must be controlled with medication, activity and diet in the A1c must be less than 10%.
- Be diagnosed with painful diabetic neuropathy. Other potential causes of neuropathy such as B12, alcohol chemotherapy, thyroid, hepatitis, and HIV must be ruled out.
- Be evaluated for peripheral vascular disease to make sure that this is not the source of pain.
- Must have tried and failed 2 or more commonly prescribed PDN medications. It is not necessary for patients to have taken opioids in order to qualify for HFX therapy.
What is the HFX process like?
Once the patient has been identified as a potential candidate for HFX therapy, they will be evaluated by a pain specialist. Appropriate testing will be completed and the patient will be scheduled for a trial of HFX. This involves placement of 2 small electrical leads in the epidural space. These are placed very carefully and precisely using x-ray guidance in the physicians’ office. Patients will typically undergo a one-week trial so that they can determine whether or not HFX therapy helps them. If there is a 50% improvement in pain or better, the patient will be offered a permanent implantation of and HFX device. This involves implantation of a small battery which can be controlled with remote control.
HFX therapy is an FDA-approved breakthrough therapy that may provide meaningful and durable pain relief in patients suffering from PDN. It is covered by most insurance policies, and should be considered if PDN is present and is negatively impacting a person’s quality of life.